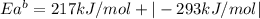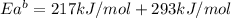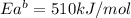Chemistry, 14.09.2019 11:10 joelpimentel
The activation energy for the reaction no2 (g )+ co (g) ⟶ no (g) + co2 (g) is ea = 217 kj/mol and the change in enthalpy for the reaction is δh = -293 kj/mol .
what is the activation energy for the reverse reaction?
enter your answer numerically and in terms of kj/mol.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which describes interactions between substances and stomata during photosynthesis? check all that apply. oxygen enters stomata. oxygen is released through stomata. carbon dioxide enters stomata. carbon dioxide is released through stomata. hydrogen enters stomata. hydrogen is released through stomata.
Answers: 1


Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1

Chemistry, 23.06.2019 09:30
Need , hurry pls create a superhero out of the element iron, what are its powers and his sidekick ( an element that works well with iron). how was the superhero made and who discovered him
Answers: 3
You know the right answer?
The activation energy for the reaction no2 (g )+ co (g) ⟶ no (g) + co2 (g) is ea = 217 kj/mol and th...
Questions




Social Studies, 28.02.2020 20:05

Mathematics, 28.02.2020 20:05









English, 28.02.2020 20:06






Mathematics, 28.02.2020 20:06

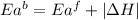
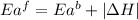
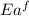 = activation energy for forward reaction
= activation energy for forward reaction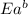 = activation energy for backward reaction
= activation energy for backward reaction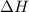 = change in enthalpy of reaction
= change in enthalpy of reaction