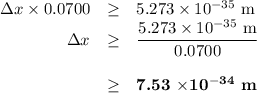Chemistry, 14.09.2019 11:30 cjstablet04
Consider an electron with a mass of 9.11 x 1051 kg and a 100.0 g tennis ball that are both moving with a velocity of 70.0 m s1. (a) calculate the momentum of the electron (p mv). (b) calculate the momentum of the tennis ball (c) what is the uncertainty in the position of the electron (ax) if the uncertainty in its momentum (ap) is equal to 1.0 % of p for the electron? (d) what is the uncertainty in the position of the tennis ball (ax) if the uncertainty in momentum (ap) is equal to 0.1% of p for the tennis ball? (e) comment on how the uncertainty in position (ax) compares to the overall size in each case

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:50
H2so4(aq) + mg(s)—> mgso4(aq) +h2(g) which substance is the acid in the reaction?
Answers: 3



Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2
You know the right answer?
Consider an electron with a mass of 9.11 x 1051 kg and a 100.0 g tennis ball that are both moving wi...
Questions



Mathematics, 12.10.2020 01:01

Mathematics, 12.10.2020 01:01

Arts, 12.10.2020 01:01

Mathematics, 12.10.2020 01:01





Mathematics, 12.10.2020 01:01

Computers and Technology, 12.10.2020 01:01


Mathematics, 12.10.2020 01:01


Mathematics, 12.10.2020 01:01

Mathematics, 12.10.2020 01:01

Mathematics, 12.10.2020 01:01

History, 12.10.2020 01:01

Mathematics, 12.10.2020 01:01