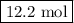Chemistry, 14.09.2019 11:30 TyleenValdez975
Calculating and using the molar mass of heterodiatomic the chemical formula for lithium fluoride is lif a chemist measured the amount of lithium fluoride produced during an experiment. she finds that 317. g of lithium fluoride is produced. calculate the number of moles of lithium fluoride produced. round your answer to 3 significant digits. mol x explanation check 2019 mcgrawh terms of education all righes reserved

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:30
100 points answer quick the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 05:30
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 13:30
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2
You know the right answer?
Calculating and using the molar mass of heterodiatomic the chemical formula for lithium fluoride is...
Questions


Mathematics, 05.10.2019 09:20

Mathematics, 05.10.2019 09:20

Mathematics, 05.10.2019 09:20

Health, 05.10.2019 09:20

Mathematics, 05.10.2019 09:20

History, 05.10.2019 09:20

History, 05.10.2019 09:20

Health, 05.10.2019 09:20


Social Studies, 05.10.2019 09:20

Physics, 05.10.2019 09:20

Mathematics, 05.10.2019 09:20

Chemistry, 05.10.2019 09:20


History, 05.10.2019 09:20


Social Studies, 05.10.2019 09:20


English, 05.10.2019 09:20