

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 1



Chemistry, 23.06.2019 04:40
Temperature is defined as a. the equivalent of heat. b. a measure of the average kinetic energy of the individual atoms or molecules composing a substance. c. how hot or cold it is. d. the total kinetic energy of the atoms or molecules composing a substance. e. none of the above is correct.
Answers: 1
You know the right answer?
Calculate the atomic weight of rubidium, which has two isotopes with the following properties: 85^r...
Questions




Geography, 28.08.2020 02:01

Mathematics, 28.08.2020 02:01



Biology, 28.08.2020 02:01

Mathematics, 28.08.2020 02:01

History, 28.08.2020 02:01

Biology, 28.08.2020 02:01

English, 28.08.2020 02:01

Mathematics, 28.08.2020 02:01

Computers and Technology, 28.08.2020 02:01

History, 28.08.2020 02:01


History, 28.08.2020 02:01

Mathematics, 28.08.2020 02:01

Physics, 28.08.2020 02:01

English, 28.08.2020 02:01

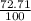 = 61.73 amu
= 61.73 amu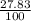 = 24.18 amu
= 24.18 amu

