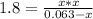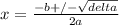Chemistry, 16.09.2019 19:30 gracebuffum
Phosphorus pentachloride decomposes according to the chemical equation pcl5(g)↽−−⇀pcl3(g)+cl2(g)kc=1.80 at 250 ∘c a 0.157 mol sample of pcl5(g) is injected into an empty 2.50 l reaction vessel held at 250 ∘c. calculate the concentrations of pcl5(g) and pcl3(g) at equilibrium.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 15:50
Nitrogen dioxide reacts with water to form nitric acid and nitrogen monoxide according to the equation: 3no2(g)+h2o(l)→2hno3(l)+no(g) part a suppose that 4.2 mol no2 and 0.50 mol h2o combine and react completely. which reactant is in excess? express your answer as a chemical formula. nothing
Answers: 1

Chemistry, 22.06.2019 01:00
What are the variables in gay-lussac’s law? pressure and volume pressure, temperature, and volume pressure and temperature volume, temperature, and moles of gas
Answers: 1

Chemistry, 22.06.2019 12:00
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2

Chemistry, 23.06.2019 00:10
Apropane torch is lit inside a hot air balloon during preflight preparations to inflate the balloon. which condition of the gas remains constant
Answers: 2
You know the right answer?
Phosphorus pentachloride decomposes according to the chemical equation pcl5(g)↽−−⇀pcl3(g)+cl2(g)kc=1...
Questions





Mathematics, 13.11.2019 17:31

World Languages, 13.11.2019 17:31








English, 13.11.2019 17:31


English, 13.11.2019 17:31

Mathematics, 13.11.2019 17:31

Chemistry, 13.11.2019 17:31

Mathematics, 13.11.2019 17:31

Mathematics, 13.11.2019 17:31

![Kc = \frac{[C]^cx[D]^d}{[A]^ax[B]^b}](/tpl/images/0233/9746/d3f86.png)
![Kc = \frac{[PCl3]x[Cl2]}{[PCl5]}](/tpl/images/0233/9746/20f28.png)