
Chemistry, 17.09.2019 00:10 jewlbug4358
Question 9 suppose 15.3g of sodium bromide is dissolved in 250.ml of a 0.40 m aqueous solution of silver nitrate. calculate the final molarity of bromide anion in the solution. you can assume the volume of the solution doesn't change when the sodium bromide is dissolved in it.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 15:00
1) how many electrons are in each energy level of the following elements? a. he b. na c. na d. ne 2) how many valence electrons are percent in the following atoms? a. s b. mg c. be d. cl 3) which of the following elements are stable as atoms? a. he b. o c. cl d. ar if you are able to provide the work as to how you got the answers that would be greatly appreciated. : )
Answers: 1



Chemistry, 22.06.2019 07:00
How many moles are in 7.2 x 10^23 carbon molecules? (*round to the nearest hundredth and include the unit "mol c" after your number) question 6 options:
Answers: 2
You know the right answer?
Question 9 suppose 15.3g of sodium bromide is dissolved in 250.ml of a 0.40 m aqueous solution of si...
Questions

Mathematics, 06.12.2021 23:20

History, 06.12.2021 23:20

Computers and Technology, 06.12.2021 23:20



Mathematics, 06.12.2021 23:20


Biology, 06.12.2021 23:20

Health, 06.12.2021 23:20

Social Studies, 06.12.2021 23:20



Mathematics, 06.12.2021 23:20

Health, 06.12.2021 23:20





History, 06.12.2021 23:20

Biology, 06.12.2021 23:20

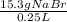 ×
× 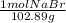 =
= 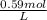 = 0.59 M
= 0.59 M

