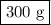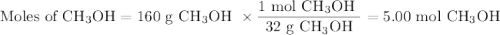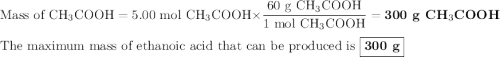The following reaction produces ethanoic acid (chacooh) from methanol (ch3oh) and carbon
monox...

Chemistry, 17.09.2019 01:00 ayeelol1447
The following reaction produces ethanoic acid (chacooh) from methanol (ch3oh) and carbon
monoxide
chcooh
ch3oh + co
relative atomie mass: h = 1: 0 = 16; c = 12
calculate the maximum mass of ethanoic acid that can be produced from 160g methanol,
assuming the carbon monoxide is in excess.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:00
Identify the electromagnets with poles that are reversed from the electromagnet shown above
Answers: 3


Chemistry, 22.06.2019 17:40
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1

Chemistry, 23.06.2019 03:40
The following questions a24 - a26 relate to 100 ml of 0.0150 m solution of benzoic acid (c6h3cooh). ka(c6h3cooh) = 6.4 x 10^-5. what is the ph of the solution after the addition of 1 x 10^-3 moles of naoh? you may assume no volume change to the solution upon addition of the naoh.
Answers: 2
You know the right answer?
Questions



Physics, 16.09.2019 06:00


History, 16.09.2019 06:00




Mathematics, 16.09.2019 06:00



History, 16.09.2019 06:00


Biology, 16.09.2019 06:00


Mathematics, 16.09.2019 06:00