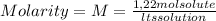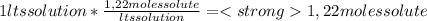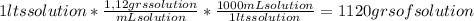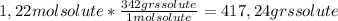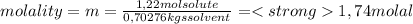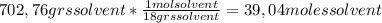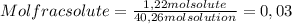Chemistry, 17.09.2019 02:00 romaguera06
A) calculatethe molality, m, of an aqueous solution of 1.22 m sucrose, c12h22o11. the density of the solution is 1.12 g/ml. b) what is the mass percent of sucrose in this solution? c) what is the mole fraction of sucrose in this solution?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:00
Different isotopes indicate that an element will have different numbers of
Answers: 2

Chemistry, 22.06.2019 00:00
How many liters of water vapor can be produced if 108 grams of methane gas (ch4) are combusted at 312 k and 0.98 atm? show all work. pls ! will mark as brainliest
Answers: 1

Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3

Chemistry, 23.06.2019 02:00
What causes the appearance of lines in a emission spectrum
Answers: 1
You know the right answer?
A) calculatethe molality, m, of an aqueous solution of 1.22 m sucrose, c12h22o11. the density of the...
Questions

History, 19.02.2021 14:00


Chemistry, 19.02.2021 14:00




Computers and Technology, 19.02.2021 14:00


Mathematics, 19.02.2021 14:00



Physics, 19.02.2021 14:00

Physics, 19.02.2021 14:00


Physics, 19.02.2021 14:00

Physics, 19.02.2021 14:00

Mathematics, 19.02.2021 14:00



Physics, 19.02.2021 14:00