use the periodic table to find molar masses.


Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 14:00
Classify each statement about effective nuclear charge, zeff, as true or false.
Answers: 2

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 08:30
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2
You know the right answer?
What mass of oxygen forms from 71.89 g co2?
use the periodic table to find molar masses.
use the periodic table to find molar masses.
Questions

Mathematics, 24.09.2019 21:30


Biology, 24.09.2019 21:30




English, 24.09.2019 21:30

Mathematics, 24.09.2019 21:30

Social Studies, 24.09.2019 21:30





Mathematics, 24.09.2019 21:30

English, 24.09.2019 21:30


History, 24.09.2019 21:30



Mathematics, 24.09.2019 21:30

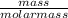
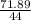 = 1.63mole
= 1.63mole

