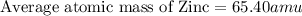2.3 zinc has five naturally occurring isotopes: 48.63% of 64 zn with an atomic weight of 63.929 amu; 27.90% of 66zn with an atomic weight of 65.926 amu; 4.10% of 67zn with an atomic weight of 66.927 amu; 18.75% of 68zn with an atomic weight of 67.925 amu; and 0.62% of 70zn with an atomic weight of 69.925 amu. calculate the average atomic weight of zn.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:10
When the volume and number of particles of a gas are constant which of the following is also constant
Answers: 3

Chemistry, 22.06.2019 13:00
Lab reagent, hypothesis test.a reference solution used as a lab reagent is purported to have a concentration of 5 mg/dl. six samples are taken from this solution and the following concentrations are recorded: (5.32, 4.88, 5.10, 4.73, 5.15, 4.75) mg/dl.these six measurements are assumed to be an srs of all possible measurements from solution.they are also assumed to have a standard deviation of 0.2, a normal distributin, and a mean concentration equal to the true concentration of the solution.carry out a significance test to determine whether these six measurements provide reliable evidence that the true concentration of the solution is actually not 5 mg/dl.
Answers: 1

Chemistry, 22.06.2019 13:30
Some animals that try to adapt to climate changes eventually die due to starvation, as climate change alters the web.
Answers: 2

Chemistry, 22.06.2019 17:30
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2
You know the right answer?
2.3 zinc has five naturally occurring isotopes: 48.63% of 64 zn with an atomic weight of 63.929 amu...
Questions



Social Studies, 10.10.2019 02:40

Biology, 10.10.2019 02:40


Computers and Technology, 10.10.2019 02:40

History, 10.10.2019 02:40

Business, 10.10.2019 02:40

Mathematics, 10.10.2019 02:40


Mathematics, 10.10.2019 02:40


Social Studies, 10.10.2019 02:40


Mathematics, 10.10.2019 02:40



History, 10.10.2019 02:40

Mathematics, 10.10.2019 02:40

Mathematics, 10.10.2019 02:40

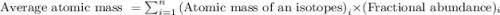 .....(1)
.....(1)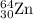 isotope:
isotope: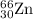 isotope:
isotope: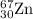 isotope:
isotope: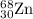 isotope:
isotope: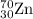 isotope:
isotope:![\text{Average atomic mass of Zinc}=[(63.929\times 0.4863)+(65.926\times 0.2790)+(66.927\times 0.0410)+(67.925\times 0.1875)+(69.925\times 0.0062)]](/tpl/images/0237/0119/27e73.png)