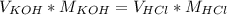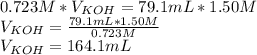Chemistry, 17.09.2019 19:00 chaseking120418
Titration is a type of experiment that can be performed to investigate a neutralization reaction. the equivalence point is when all of the acid and base is fully neutralized. a sample of 0.723 m aqueous potassium hydroxide was titrated against a standard solution of hydrochloric acid. what was the volume of the potassium hydroxide solution if 79.1 ml of 1.50 m hydrochloric acid was needed to reach the equivalence point?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:00
Noble gases are the most reactive elements on the periodic table. a. true b. false
Answers: 2

Chemistry, 23.06.2019 06:00
If you try to move a piano and are unable to move it, did you perform any work in the scientific sense of the word? yes? or no? this question is worth 20 points! let it be correct!
Answers: 1

Chemistry, 23.06.2019 06:00
When hydrogen peroxide (h2o2) is added to potassium iodide (ki) solution, the hydrogen peroxide decomposes into water (h2o) and oxygen (o2). the chemical equation for the decomposition reaction is: 2h2o2—> 2h2o + o2. what is the role of the potassium iodide in this reaction? a. reactant. b. product. c. precipitate. d. catalyst.
Answers: 1

Chemistry, 23.06.2019 06:30
Which of these describes how heat is transferred by convection* a. sunlight travels through space without the aid of fluids or solids. b. warm air rises and takes the heat with it, eventually, it cools and sinks c. air at the equator rises and sinks at the poles. d. air molecules touch the warm ground, heating them up *not conduction
Answers: 3
You know the right answer?
Titration is a type of experiment that can be performed to investigate a neutralization reaction. th...
Questions


Chemistry, 30.10.2021 14:00

Mathematics, 30.10.2021 14:00


Mathematics, 30.10.2021 14:00

Mathematics, 30.10.2021 14:00

Mathematics, 30.10.2021 14:00








Mathematics, 30.10.2021 14:00

SAT, 30.10.2021 14:00


Mathematics, 30.10.2021 14:00

English, 30.10.2021 14:00

Mathematics, 30.10.2021 14:00