
Iron reacts with oxygen to produce iron(iii) oxide as represented above. a 75.0 g sample of fe(s)is mixed with 11.5 l of o2(g) at 2.66 atm and 298 k.(a) calculate the number of moles of each of the following before the reaction occurs.(i) fe(s)(ii) o2(g)(b) identify the limiting reactant when the mixture is heated to produce fe2o3. support youranswer with calculations.(c) calculate the number of moles of fe2o3 produced when the reaction proceeds tocompletion.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 08:00
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 10:30
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1

Chemistry, 22.06.2019 21:50
28. which is not a reason that water is used to store spent fuel rods from nuclear power plants? water increases the speed of the chain reaction in the fuel rods. water protects nuclear power plant workers from the high temperature and radiation of the fuel rods. water acts as a radiation shield to reduce the radiation levels. water cools the spent rods. salts action
Answers: 1
You know the right answer?
Iron reacts with oxygen to produce iron(iii) oxide as represented above. a 75.0 g sample of fe(s)is...
Questions


Business, 29.10.2019 07:31



History, 29.10.2019 07:31


History, 29.10.2019 07:31







Mathematics, 29.10.2019 07:31

English, 29.10.2019 07:31

English, 29.10.2019 07:31

Mathematics, 29.10.2019 07:31



Mathematics, 29.10.2019 07:31

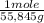 = 1,34 Fe(s) moles
= 1,34 Fe(s) moles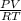 = n
= n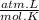
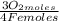 = 1,01 O₂ moles
= 1,01 O₂ moles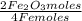 =
= 

