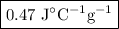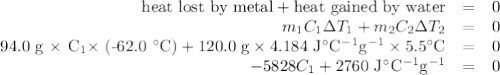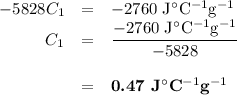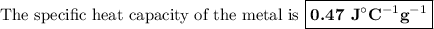A74.0-gram piece of metal at 94.0 °c is placed in 120.0 g of water in a calorimeter at 26.5 °c. the final temperature in the calorimeter is 32.0 °c. determine the specific heat of the metal. show your work by listing various steps, and explain how the law of conservation of energy applies to this situation.

Answers: 1
Another question on Chemistry

Chemistry, 20.06.2019 18:02
Heat by radiation will most likely be transferred through a.gas b.liquid c.solid d.insulator
Answers: 1

Chemistry, 22.06.2019 01:20
Match the acid base pairs by arranging the acid name with the conjugate base formula. hydrogen carbonate hydrogen phosphate carbonic acid read water sulfuric acid phosphoric acid a. co32- b. hso4- c. hco3- d. po43- e. h2po4- f. oh-
Answers: 1

Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 22.06.2019 14:30
Is a pencil falling to the floor anon contact force, a force, or a contact force
Answers: 1
You know the right answer?
A74.0-gram piece of metal at 94.0 °c is placed in 120.0 g of water in a calorimeter at 26.5 °c. the...
Questions



Mathematics, 02.06.2020 03:58



Mathematics, 02.06.2020 03:58









English, 02.06.2020 03:58

Mathematics, 02.06.2020 03:58

Mathematics, 02.06.2020 03:59


Chemistry, 02.06.2020 03:59

English, 02.06.2020 03:59