
Chemistry, 17.09.2019 21:20 DivineMemes420
You are asked by the king of allegesia to determine if the crown he was given for his birthday is pure gold. the density of pure gold is 19.32 g/ml; the density of water is 0.998 g/ml at 20ºc. there is a container when filled to the mark contains 1900.0 ml. when the container is filled with water to the mark, it has a mass of 2499.5 g. the crown is placed in the container and filled to the mark with water and now weighs 15,113.3 g. the crown has a mass of 13,910.0 g. what is the density of the crown? is the crown pure gold?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:30
When an object falls through the air and encounters air resistance its overall speed will be than if it had not encountered air resistance? (one word answer)
Answers: 2

Chemistry, 22.06.2019 07:10
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2

Chemistry, 22.06.2019 21:00
As we move from left to right across the periodic table, what is the general trend? a) atomic radii increase. b) electronegavitiy decreases. c) nuclear shielding increases. d) metallic character decreases.
Answers: 1

Chemistry, 23.06.2019 03:30
If 2 molecules of one reactant combine with 3 molecules of another to produce 5 molecules of a product, then what is the representation of the reaction?
Answers: 1
You know the right answer?
You are asked by the king of allegesia to determine if the crown he was given for his birthday is pu...
Questions


History, 13.07.2019 11:00

English, 13.07.2019 11:00

Mathematics, 13.07.2019 11:00



Mathematics, 13.07.2019 11:00

History, 13.07.2019 11:00




Physics, 13.07.2019 11:00

Mathematics, 13.07.2019 11:00







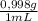 = 1896,2 g of water
= 1896,2 g of water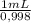 = 601,2 mL
= 601,2 mL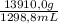 = 10,71 g/mL
= 10,71 g/mL

