
Amixture of noble gases [helium (mw 4), argon (mw 40), krypton (mw 83.8), and xenon (mw 131.3)] is at a total pressure of 150 kpa, and a temperature of 500 k. the mixture has the following composition in mole fraction: 0.25 helium, 0.25 argon, 0.25 krypton. determine: (a) the mass fraction of helium. (b) the average molecular weight of the mixture. (c) the total molar concentration. (d) the mass density.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2


Chemistry, 23.06.2019 08:00
Pl what kind of reaction is this? nahco3 + h2o → co2 + naoh + h2o -composition -decomposition -single replacement -double replacement im leaning more toward single replacement. if im wrong can you explain whyy?
Answers: 2
You know the right answer?
Amixture of noble gases [helium (mw 4), argon (mw 40), krypton (mw 83.8), and xenon (mw 131.3)] is a...
Questions



Chemistry, 18.04.2021 04:50

History, 18.04.2021 04:50




Mathematics, 18.04.2021 04:50



Mathematics, 18.04.2021 04:50

Mathematics, 18.04.2021 04:50

Computers and Technology, 18.04.2021 04:50

Physics, 18.04.2021 04:50



Mathematics, 18.04.2021 04:50


History, 18.04.2021 04:50

Mathematics, 18.04.2021 04:50

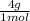 = 1 g of HeliumArgon: 0,25 moles ×
= 1 g of HeliumArgon: 0,25 moles ×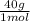 = 10 g of ArgonKrypton: 0,25 moles ×
= 10 g of ArgonKrypton: 0,25 moles ×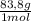 = 20,95 g of krypton Xenon: 0,25 moles ×
= 20,95 g of krypton Xenon: 0,25 moles ×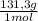 = 32,825 g of Xenon
= 32,825 g of Xenon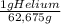 × 100 = 1,6%
× 100 = 1,6%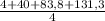 = 64,775 g/mol
= 64,775 g/mol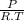 = M
= M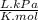
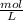 ×
× 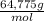 ×
× 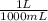 =
=

