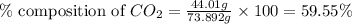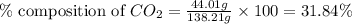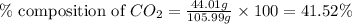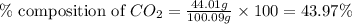A1.1000 gram carbonate sample chosen from li2co3, k2co3, na2co3 and caco3 was reacted with h2so4 and was found to lose 0.3497 gram of co2. (1) show the calculation of the % co2 in the unknown carbonate sample. (2) show the calculation of the % co2 in each of the carbonate compounds and identify the unknown carbonate from the list. atomic weights: c = 12.01, o = 16.00. mws: li2co3 = 73.892, k2co3 = 138.21, na2co3 = 105.99 and caco3 = 100.09

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 16:20
Aluminum reacts with chlorine gas to form aluminum chloride via the following reaction: 2al(s)+3cl2(g)→2alcl3(s) what is the maximum mass of aluminum chloride that can be formed when reacting 32.0 g of aluminum with 37.0 g of chlorine? express your answer to three significant figures and include the appropriate units.
Answers: 2

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

Chemistry, 22.06.2019 23:30
What are the similarities between compounds and mixtures?
Answers: 3

Chemistry, 23.06.2019 01:00
Aman applies a force of 500n to push a truck 100m down the street how much does he do?
Answers: 1
You know the right answer?
A1.1000 gram carbonate sample chosen from li2co3, k2co3, na2co3 and caco3 was reacted with h2so4 and...
Questions


Mathematics, 28.01.2020 14:49

Arts, 28.01.2020 14:49



History, 28.01.2020 14:49




Mathematics, 28.01.2020 14:49

English, 28.01.2020 14:49

Social Studies, 28.01.2020 14:50


Mathematics, 28.01.2020 14:50

Social Studies, 28.01.2020 14:50

Mathematics, 28.01.2020 14:50

Mathematics, 28.01.2020 14:50



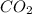 in unknown carbonate sample is 31.79 %
in unknown carbonate sample is 31.79 %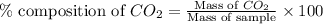 .....(1)
.....(1)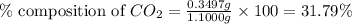
![[1\times 12.01)+(2\times 16.00)]=44.01g](/tpl/images/0237/8770/41a6a.png)