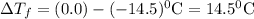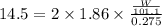Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at −14.5 ∘c? the freezing point for pure water is 0.0 ∘c and kf is equal to 1.86 ∘c/m. express your answer to three significant figures and include the appropriate units.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 15:40
What is the study of how matter and energy interact? a. biology b. physics c. planetary science d. chemistry
Answers: 1

Chemistry, 21.06.2019 16:30
In which direction will the following reaction go if the standard reduction potentials are 0.80 v for ag/ag+ and –0.44 v for fe/fe2+? ag+ + fe → ag + fe2+ a.)forward b.)the reaction cannot occur. c.) not enough information is given. d.) reverse
Answers: 1


Chemistry, 22.06.2019 03:00
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2
You know the right answer?
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of wate...
Questions


Mathematics, 21.05.2020 22:57


Computers and Technology, 21.05.2020 22:57



Mathematics, 21.05.2020 22:57

Mathematics, 21.05.2020 22:57





Mathematics, 21.05.2020 22:57

Mathematics, 21.05.2020 22:57




Mathematics, 21.05.2020 22:57


Mathematics, 21.05.2020 22:57

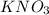 must be added
must be added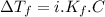
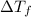 is depression in freezing point of solution, i is van't hoff factor (equal to number of ions produce from dissociation of 1 molecule of electrolyte) and C is molality of solutionMolar mass of
is depression in freezing point of solution, i is van't hoff factor (equal to number of ions produce from dissociation of 1 molecule of electrolyte) and C is molality of solutionMolar mass of 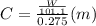 For
For 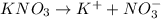 )Here
)Here