
Chemistry, 18.09.2019 01:00 emalvidrez5205
Consider the following reaction: chcl3(g) + cl2(g) → ccl4(g) + hcl(g) the initial rate of the reaction is measured at several different concentrations of the reactants with the following results: [chcl3] (m) [cl2] (m) initial rate (m/s) 0.010 0.010 0.0035 0.020 0.010 0.0069 0.020 0.020 0.0098 0.040 0.040 0.027 from the data, choose the correct rate law for the reaction. rate=k[chcl3][cl2]2 rate=k[chcl3][cl2]12 rate=k[chcl3]2[cl2] rate=k[chcl3]12[cl2]

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:00
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1

Chemistry, 22.06.2019 09:50
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1

Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2

You know the right answer?
Consider the following reaction: chcl3(g) + cl2(g) → ccl4(g) + hcl(g) the initial rate of the react...
Questions

Mathematics, 05.02.2021 22:40

Mathematics, 05.02.2021 22:40




Social Studies, 05.02.2021 22:40

Health, 05.02.2021 22:40

German, 05.02.2021 22:40

Mathematics, 05.02.2021 22:40


Arts, 05.02.2021 22:40

Social Studies, 05.02.2021 22:40

History, 05.02.2021 22:40

Mathematics, 05.02.2021 22:40

Mathematics, 05.02.2021 22:40


Social Studies, 05.02.2021 22:40

Mathematics, 05.02.2021 22:40

Biology, 05.02.2021 22:40

History, 05.02.2021 22:40

![\text{Rate}=k[CHCl_3][Cl_2]^{1/2}](/tpl/images/0238/0898/b5249.png)
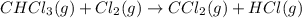
![\text{Rate}=k[CHCl_3]^a[Cl_2]^b](/tpl/images/0238/0898/f7b12.png)
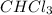
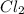
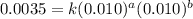 ....(1)
....(1)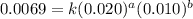 ....(2)
....(2)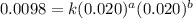 ....(3)
....(3)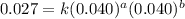 ....(4)
....(4)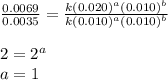
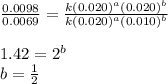
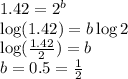
![\text{Rate}=k[CHCl_3]^1[Cl_2]^{1/2}](/tpl/images/0238/0898/0ea6b.png)


