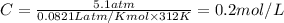The osmotic pressure exerted by a solution is equal to the molarity multiplied by the absolute temperature and the gas constant r. suppose the osmotic pressure of a certain solution is measured to be 5.1 atm at an absolute temperature of 312 k. write an equation that will let you calculate the molarity of this solution. your equation should contain only symbols. be sure you define each symbol other than r .

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:00
Which process does not require the presence of a physical substance in order to transfer heat? air in the atmosphere is heated by the ground. this warm air then rises, and cooler air falls. this is an example of what type of process? how is conduction different from radiation?
Answers: 1

Chemistry, 22.06.2019 15:00
Which substance is a steroid? cholesterol fatty acid monosaccharide trans fat
Answers: 1

Chemistry, 22.06.2019 20:30
Citric acid has a ph between 1 and 3. it is considered to be aa. weak acidb. weak basec. strong based. strong acid
Answers: 2

You know the right answer?
The osmotic pressure exerted by a solution is equal to the molarity multiplied by the absolute tempe...
Questions

Mathematics, 22.08.2019 21:30

Mathematics, 22.08.2019 21:30

World Languages, 22.08.2019 21:30

Geography, 22.08.2019 21:30

Mathematics, 22.08.2019 21:30

Mathematics, 22.08.2019 21:30

Biology, 22.08.2019 21:30





Mathematics, 22.08.2019 21:30


Biology, 22.08.2019 21:30


Mathematics, 22.08.2019 21:30



Health, 22.08.2019 21:30

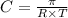

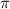 = osmotic pressure = 5.1 atm
= osmotic pressure = 5.1 atm