
Chemistry, 18.09.2019 04:00 Elepeodowke
Determine the concentrations of mgcl2, mg2+, and cl− in a solution prepared by dissolving 2.852.85 × 10−4 g mgcl2 in 2.252.25 l of water. express all three concentrations in molarity. additionally, express the concentrations of the ionic species in parts per million (ppm).

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:30
Joan writes four numbers on the board in standard form, and then she writes their scientific notation
Answers: 1

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

You know the right answer?
Determine the concentrations of mgcl2, mg2+, and cl− in a solution prepared by dissolving 2.852.85 ×...
Questions


Mathematics, 16.10.2019 06:30

English, 16.10.2019 06:30

Mathematics, 16.10.2019 06:30

Mathematics, 16.10.2019 06:30


History, 16.10.2019 06:30




Mathematics, 16.10.2019 06:30

Biology, 16.10.2019 06:30


Geography, 16.10.2019 06:30

History, 16.10.2019 06:30



Mathematics, 16.10.2019 06:30

History, 16.10.2019 06:30

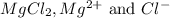 are 0.127 ppm, 0.127 ppm and 0.254 ppm respectively.
are 0.127 ppm, 0.127 ppm and 0.254 ppm respectively.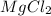 =
= 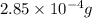
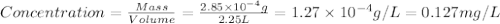
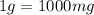
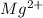 and
and 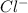 .
.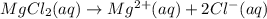
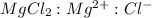 is, 1 : 1 : 2. So,
is, 1 : 1 : 2. So,

