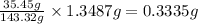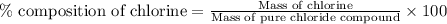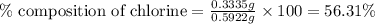Chemistry, 18.09.2019 03:30 shortcake8047
A0.5922 g sample of a pure soluble chloride compound is dissolved in water, and all of the chloride ion is precipitated as agcl by the addition of an excess of silver nitrate. the mass of the resulting agcl is found to be 1.3487 g. what is the mass percentage of chlorine in the original compound?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 16:30
10-14. (a) when 100.0 ml of weak acid ha were titrated with 0.093 81 m naoh, 27.63 ml were required to reach the equivalence point. find the molarity of ha. (b) what is the formal concentration of a- at the equivalence point? (c) the ph at the equivalence point was 10.99. find pk. for ha. (d) what was the ph when only 19.47 ml of naoh had been added?
Answers: 1

Chemistry, 22.06.2019 02:30
Needthe meter is the standard unit for: 1) height 2) length 3) weight 4) mass
Answers: 3

Chemistry, 22.06.2019 08:30
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2

Chemistry, 22.06.2019 15:30
How does a large body of water, such as the ocean, influence climate?
Answers: 1
You know the right answer?
A0.5922 g sample of a pure soluble chloride compound is dissolved in water, and all of the chloride...
Questions


Physics, 14.01.2022 07:30



Social Studies, 14.01.2022 07:30



Physics, 14.01.2022 07:30

Mathematics, 14.01.2022 07:30


Mathematics, 14.01.2022 07:30



English, 14.01.2022 07:30




Chemistry, 14.01.2022 07:30

English, 14.01.2022 07:30