Chemistry, 18.09.2019 03:30 Ruthsybel9754
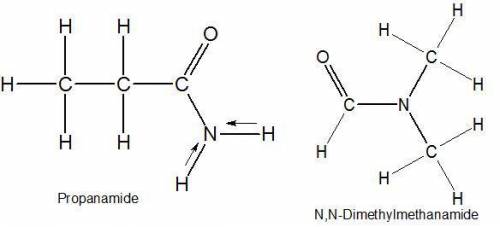
Select the single best answer. why is the boiling point of propanamide, ch3ch2conh2, considerably higher than the boiling point of n, n−dimethylformamide, hcon(ch3)2 (213°c vs. 153°c), even though both compounds are isomeric amides? ch3ch2conh2 has weaker intermolecular forces than hcon(ch3)2 hydrogen bonding is present in ch3ch2conh2 but not in hcon(ch3)2 ch3ch2conh2 is more branched than hcon(ch3)2 ch3ch2conh2 has two methyl groups

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1

Chemistry, 22.06.2019 10:30
How do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 2

Chemistry, 22.06.2019 16:00
Which process transfers heat from inside earth to its surface? convection currents in mantle pulling away of tectonic plates drawing in of tectonic plates convection currents in crust
Answers: 1
You know the right answer?
Select the single best answer. why is the boiling point of propanamide, ch3ch2conh2, considerably hi...
Questions


History, 06.05.2020 08:58


History, 06.05.2020 08:58


Mathematics, 06.05.2020 08:58


Mathematics, 06.05.2020 08:58



English, 06.05.2020 08:58


World Languages, 06.05.2020 08:58




History, 06.05.2020 08:58

History, 06.05.2020 08:58


Mathematics, 06.05.2020 08:58