
Chemistry, 18.09.2019 04:10 maddie7155
At elevated temperatures, dinitrogen pentoxide decomposes to nitrogen dioxide and oxygen: 2n2o5(g) → 4no2 (g) + o2 (g) when the rate of formation of no2 is 5.5 ⋅ 10-4 m/s, the rate of decomposition of n2o5 is m/s. at elevated temperatures, dinitrogen pentoxide decomposes to nitrogen dioxide and oxygen: 2n2o5(g) 4no2 (g) + o2 (g) when the rate of formation of no2 is 5.5 10-4 m/s, the rate of decomposition of n2o5 is m/s. 2.8 ⋅ 10-4 1.4 ⋅ 10-4 5.5 ⋅ 10-4 10.1 ⋅ 10-4 2.2 ⋅ 10-3

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1

Chemistry, 22.06.2019 22:00
What mass of glucose is produced when 54g of water react with carbon dioxide
Answers: 1

Chemistry, 23.06.2019 06:20
Why is it that 85.48 rounded to two significant figures is 85 and not 86?
Answers: 1
You know the right answer?
At elevated temperatures, dinitrogen pentoxide decomposes to nitrogen dioxide and oxygen: 2n2o5(g)...
Questions

Geography, 23.09.2020 14:01




Chemistry, 23.09.2020 14:01


Chemistry, 23.09.2020 14:01


Business, 23.09.2020 14:01

History, 23.09.2020 14:01



Chemistry, 23.09.2020 14:01



History, 23.09.2020 14:01


Mathematics, 23.09.2020 14:01


Mathematics, 23.09.2020 14:01

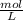 -
-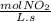 ×
× 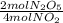 = 2,8 M/s
= 2,8 M/s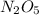 is
is 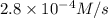
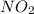 =
= 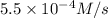
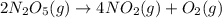
![\text{Rate of decomposition of }N_2O_5=-\frac{1}{2}\times \frac{d[N_2O_5]}{dt}](/tpl/images/0238/6091/c0940.png)
![\text{Rate of formation of }NO_2=+\frac{1}{4}\frac{d[NO_2]}{dt}](/tpl/images/0238/6091/2ef8c.png)
![\text{Rate of formation of }O_2=+\frac{d[O_2]}{dt}](/tpl/images/0238/6091/db5e4.png)
![\text{Rate of reaction}=-\frac{1}{2}\times \frac{d[N_2O_5]}{dt}=+\frac{1}{4}\frac{d[NO_2]}{dt}=+\frac{d[O_2]}{dt}](/tpl/images/0238/6091/52065.png)
![-\frac{1}{2}\times \frac{d[N_2O_5]}{dt}=+\frac{1}{4}\frac{d[NO_2]}{dt}](/tpl/images/0238/6091/5fd92.png)
![-\frac{d[N_2O_5]}{dt}=+\frac{2}{4}\frac{d[NO_2]}{dt}](/tpl/images/0238/6091/ff940.png)
![-\frac{d[N_2O_5]}{dt}=\frac{2}{4}\times (5.5\times 10^{-4}M/s)](/tpl/images/0238/6091/9cde4.png)
![-\frac{d[N_2O_5]}{dt}=2.8\times 10^{-4}M/s](/tpl/images/0238/6091/65aeb.png)


