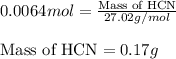Chemistry, 18.09.2019 04:10 potaetoo1997
When potassium cyanide (kcn) reacts with acids, a deadly poisonous gas, hydrogen cyanide (hcn), is given off: kcn(aq) + hcl(aq) → hcn(g) + kcl(aq) if a sample of 0.420 g of kcn is treated with an excess of hcl, calculate the amount of hcn formed in grams.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 14:10
How does chemistry affect our world? a. chemicals makes our world more polluted. b. chemicals keeps us healthy. c. chemicals can or hurt our world. d. chemicals make our world safe to live in.
Answers: 1


Chemistry, 22.06.2019 16:30
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1

Chemistry, 22.06.2019 22:10
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1
You know the right answer?
When potassium cyanide (kcn) reacts with acids, a deadly poisonous gas, hydrogen cyanide (hcn), is g...
Questions





History, 19.05.2020 20:58








History, 19.05.2020 20:59


Mathematics, 19.05.2020 20:59


Mathematics, 19.05.2020 20:59

Biology, 19.05.2020 20:59


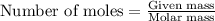 .....(1)
.....(1)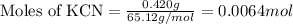
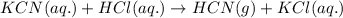
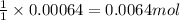 of hydrogen cyanide
of hydrogen cyanide