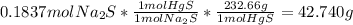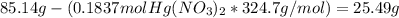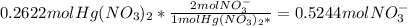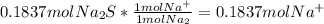Fa solution containing 85.14 g of mercury(ii) nitrate is allowed to react completely with a solution containing 14.334 g of sodium sulfide, how many grams of solid precipitate will be formed? mass: 29.69 g how many grams of the reactant in excess will remain after the reaction? mass: g assuming complete precipitation, how many moles of each ion remain in solution? if an ion is no longer in solution, enter a zero (0) for the number of moles. hg2+ : 0 mol no–3 : 0 mol na+ : 0 mol s2− : 0 mol

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 22.06.2019 10:50
A100 kmol/h stream that is 97 mole% carbon tetrachloride (ccl4) and 3% carbon disulfide (cs2) is to be recovered from the bottom of a distillation column. the feed to the column is 16 mole% cs2 and 84% ccl4, and 2% of the ccl4 entering the column is contained in the overhead stream leaving the top of the column. calculate the mass and mole fractions of ccl4 in the overhead stream, and determine the molar flow rates of ccl4 and cs2 in the overhead and feed streams. 12. mw_ccla- 153.82; mw_cs2-76.14.
Answers: 3

You know the right answer?
Fa solution containing 85.14 g of mercury(ii) nitrate is allowed to react completely with a solution...
Questions

Mathematics, 18.04.2020 21:50

Mathematics, 18.04.2020 21:50


Social Studies, 18.04.2020 21:50


English, 18.04.2020 21:50



Computers and Technology, 18.04.2020 21:50


Social Studies, 18.04.2020 21:50

Mathematics, 18.04.2020 21:50

History, 18.04.2020 21:50

Mathematics, 18.04.2020 21:50

Health, 18.04.2020 21:50


Spanish, 18.04.2020 21:50

Mathematics, 18.04.2020 21:50


History, 18.04.2020 21:50

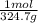 =0.2622 moles.Moles of sodium sulfide = 14.334 g *
=0.2622 moles.Moles of sodium sulfide = 14.334 g *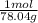 =0.1837 moles.
=0.1837 moles.