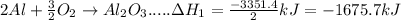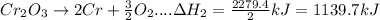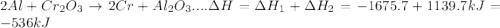In the following overall chemical reaction, aluminum displaces chromium from chromium(iii) oxide and forms aluminum oxide.
2 al(s) + cr2o3(s) → 2 cr(s) + al2o3(s); δh = ?
find the change in enthalpy for this reaction, using hess' law and the enthalpy changes for the reactions given below.
(1a) 4 al(s) + 3 o2(g) → 2 al2o3(s); δh = −3351.4 kj
(2a) 4 cr(s) + 3 o2(g) → 2 cr2o3(s); δh = −2279.4 kj

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:20
What are the spectator ions in 2h+ + so42- + ca2+ + 2r → caso4 + 2h+ + 21?
Answers: 1

Chemistry, 22.06.2019 06:00
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2

Chemistry, 22.06.2019 07:40
The formation of a solid, also known as a is an indication of a chemical change. precipitate particulate particle powder
Answers: 3

Chemistry, 23.06.2019 01:50
Ablock of aluminum is dropped into a graduated cylinder with an initial volume of water at 75ml and the volumes rises to 90ml. if the block has a mass of 40.5 g what is its density ?
Answers: 1
You know the right answer?
In the following overall chemical reaction, aluminum displaces chromium from chromium(iii) oxide and...
Questions

Mathematics, 22.02.2021 23:50

Mathematics, 22.02.2021 23:50


Mathematics, 22.02.2021 23:50

Spanish, 22.02.2021 23:50

Mathematics, 22.02.2021 23:50


History, 22.02.2021 23:50

Mathematics, 22.02.2021 23:50

Mathematics, 22.02.2021 23:50

English, 22.02.2021 23:50

Mathematics, 22.02.2021 23:50

Mathematics, 23.02.2021 01:00


Computers and Technology, 23.02.2021 01:00

Computers and Technology, 23.02.2021 01:00




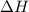 is an additive property. hence value of
is an additive property. hence value of