Chemistry, 18.09.2019 05:20 stephaniero6
Asample of liquefied natural, lng, from alaska has the following molar composition: 3.9% c2h6, 1.1% c3h8, 1.1% co2, and the rest ch4. determine (a) the average molecular weight of the lng mixture. (b) the weight fraction of ch4 in the mixture. (c) the lng is heated to 422 k and 148 kpa, and vaporizes completely. estimate the density of the gas mixture under these conditions.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:50
In a popular classroom demonstration, solid sodium is added to liquid water and reacts to produce hydrogen gas and aqueous sodium hydroxide. part a write a balanced chemical equation for this reaction. express your answer as a chemical equation. identify all of the phases in your answer.
Answers: 3


Chemistry, 22.06.2019 23:30
The density of benzene at 15 °c is 0.8787 g/ml. calculate the mass of 0.1500 l of benzene at this temperature. enter your answer in terms of grams
Answers: 2

You know the right answer?
Asample of liquefied natural, lng, from alaska has the following molar composition: 3.9% c2h6, 1.1%...
Questions











Mathematics, 16.02.2022 14:00

SAT, 16.02.2022 14:00

Spanish, 16.02.2022 14:00

Chemistry, 16.02.2022 14:00





Mathematics, 16.02.2022 14:00

SAT, 16.02.2022 14:00

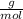
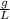
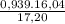 ×100 = 87,57%
×100 = 87,57%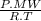 = δ
= δ