Chemistry, 18.09.2019 05:30 asuhdude57
A245.7g sample of metal at 75.0℃ was placed in 115.4g of water at 22.0℃. the final temperature of the water and metal was 34.0℃. if no heat was lost to the surroundings, what is the specific heat of the metal? (specific heat of water = 4.184 j/g℃)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which of the following is always a reactant in a combustion reaction? oxygen nitrogen hydrogen carbon
Answers: 1

Chemistry, 22.06.2019 04:30
The big bang nucleosynthesis theory states that elements were produced in the first few minutes of the big bang while elements have their origins in the interiors of stars, forming much later in the history of the universe.
Answers: 1


Chemistry, 22.06.2019 09:30
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1
You know the right answer?
A245.7g sample of metal at 75.0℃ was placed in 115.4g of water at 22.0℃. the final temperature of th...
Questions

History, 24.06.2019 23:00




Biology, 24.06.2019 23:00




Mathematics, 24.06.2019 23:00



English, 24.06.2019 23:00

English, 24.06.2019 23:00


Biology, 24.06.2019 23:00

Mathematics, 24.06.2019 23:00

Mathematics, 24.06.2019 23:00

Mathematics, 24.06.2019 23:00


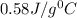
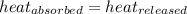
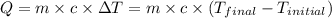
![m_1\times c_1\times (T_{final}-T_1)=-[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/0238/8184/09236.png) .................(1)
.................(1)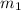 = mass of metal = 245.7 g
= mass of metal = 245.7 g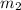 = mass of water = 115.4 g
= mass of water = 115.4 g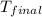 = final temperature =
= final temperature = 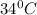
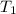 = temperature of metal =
= temperature of metal = 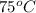
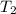 = temperature of water =
= temperature of water = 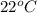
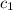 = specific heat of metal = ?
= specific heat of metal = ?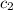 = specific heat of water=
= specific heat of water= 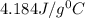
![-245.7\times c\times (75-34)=[115.4\times 4.184\times (34-22)]](/tpl/images/0238/8184/37846.png)