
Chemistry, 18.09.2019 05:30 wowihavefun
A1.0 l sample of an aqueous solution contains 0.10 mol of nacl and 0.10 mol of cacl2. what is the minimum number of moles of agno3 that must be added to the solution in order to precipitate all of the cl- as agcl(s) ? (assume that agcl is insoluble.)

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 22.06.2019 17:30
Oil rich countries in the middle east cover about 4% of earths total land area but prossess about 48% of the worlds known oil reserves what is the main reason for high concentration of reserves in this part of the world
Answers: 3

Chemistry, 22.06.2019 19:10
Which statement correctly describes the phosphate ion, ? it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge on the phosphorus atom. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge on the phosphorus atom.
Answers: 3
You know the right answer?
A1.0 l sample of an aqueous solution contains 0.10 mol of nacl and 0.10 mol of cacl2. what is the mi...
Questions


Mathematics, 21.10.2020 22:01

Mathematics, 21.10.2020 22:01


History, 21.10.2020 22:01


Computers and Technology, 21.10.2020 22:01




Chemistry, 21.10.2020 22:01



Mathematics, 21.10.2020 22:01

Arts, 21.10.2020 22:01


Spanish, 21.10.2020 22:01

History, 21.10.2020 22:01

Mathematics, 21.10.2020 22:01

 to precipitate all of the
to precipitate all of the 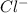 ions.
ions.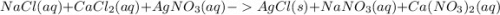
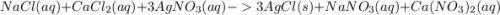
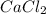 , we need 3 moles of
, we need 3 moles of 


