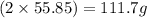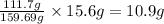Chemistry, 18.09.2019 17:10 michellemonroe012305
A29.7 g sample of iron ore is treated as follows. the iron in the sample is all converted by a series of chemical reactions to fe₂o₃. the mass of fe₂o₃ is measured to be 15.6 g. what was the mass of iron in the sample of ore? answer in units of g.

Answers: 1
Another question on Chemistry

Chemistry, 23.06.2019 00:00
#20 which type of bond is formed when bases pair in dna? ionic bond covalent bond coordinate bond hydrogen bond
Answers: 1

Chemistry, 23.06.2019 12:30
You have 125 g of a certain seasoning and are told that it contains 70.0 g of salt. what is the peroentage of salt by mass in this seasoning?
Answers: 2

Chemistry, 23.06.2019 13:00
How long could you survive without electricity? what parts of your life would be affected by loss of electricity? should you prepare for an electricity outage, and if so, how would you prepare? what backup system could your family or community install to generate limited amounts of electricity during an outage? how does this system create an electric force field and generate electric current?
Answers: 2

Chemistry, 23.06.2019 14:00
What is the final volume in milliliters when 0.641 l of a 34.0 % (m/v) solution is diluted to 23.5 % (m/v)?
Answers: 1
You know the right answer?
A29.7 g sample of iron ore is treated as follows. the iron in the sample is all converted by a serie...
Questions


Mathematics, 19.04.2021 21:50


Mathematics, 19.04.2021 21:50

History, 19.04.2021 21:50



Mathematics, 19.04.2021 21:50



Spanish, 19.04.2021 21:50


Mathematics, 19.04.2021 21:50

Mathematics, 19.04.2021 21:50




Mathematics, 19.04.2021 21:50

History, 19.04.2021 21:50

Mathematics, 19.04.2021 21:50