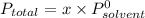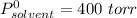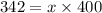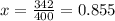Chemistry, 18.09.2019 17:20 lindseyreneesmith7
Diethyl ether has a vapor pressure of 400.0 torr at 18°c. when a sample of benzoic acid (a non-volatile compound) is dissolved in ether, the vapor pressure of the solution is 342 torr. what is the mole fraction of benzoic acid in the solution?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:30
Sex cells from female reproductive organ? 1) mitosis 2) fertilization 3) zygote 4) eggs 5) meiosis 6) sperm
Answers: 2

Chemistry, 22.06.2019 02:40
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3

Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2

You know the right answer?
Diethyl ether has a vapor pressure of 400.0 torr at 18°c. when a sample of benzoic acid (a non-volat...
Questions


Social Studies, 16.01.2020 22:31

Mathematics, 16.01.2020 22:31







Mathematics, 16.01.2020 22:31






Computers and Technology, 16.01.2020 22:31