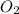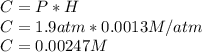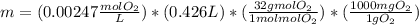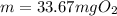Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3


Chemistry, 23.06.2019 11:30
How do you calculate the mass of a product when the amounts of more than one reactant are given?
Answers: 3

Chemistry, 23.06.2019 13:20
Use the periodic table to answer the following questions. what is the predicted order of first ionization energies from highest to lowest for beryllium, calcium, magnesium, and strontium? o be > ca > mg > sr o be > mg > ca > sr o ca > sr> be > mg o sr > ca > mg > be done
Answers: 1
You know the right answer?
What mass of oxygen gas, in mg, is dissolved in 4.26×102 ml of water when the partial pressure of ox...
Questions

History, 22.07.2021 14:00


English, 22.07.2021 14:00




World Languages, 22.07.2021 14:00





Mathematics, 22.07.2021 14:00

History, 22.07.2021 14:00



English, 22.07.2021 14:00


Social Studies, 22.07.2021 14:00


SAT, 22.07.2021 14:00