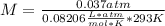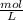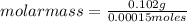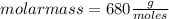Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1

Chemistry, 22.06.2019 17:40
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1

Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1

Chemistry, 23.06.2019 02:40
How can a mixture of salt water be separated into salt and water
Answers: 1
You know the right answer?
Asolution containing 0.102 g of an unknown compound dissolved in 100. ml of water has an osmotic pre...
Questions

Mathematics, 05.12.2021 01:50




Social Studies, 05.12.2021 01:50

English, 05.12.2021 01:50

Mathematics, 05.12.2021 01:50


Mathematics, 05.12.2021 01:50

Mathematics, 05.12.2021 01:50



Chemistry, 05.12.2021 01:50

Mathematics, 05.12.2021 01:50

Mathematics, 05.12.2021 01:50

Physics, 05.12.2021 01:50



Chemistry, 05.12.2021 01:50

Mathematics, 05.12.2021 01:50

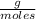
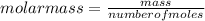
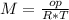
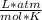 T=20 °C= 293°K (0°C=273 °K)
T=20 °C= 293°K (0°C=273 °K)