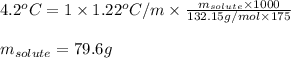Chemistry, 18.09.2019 21:20 tatyanaknight122
Cinnamaldehyde (mm = 132.15 g/mol) is used as a flavoring agent. what mass of cinnamaldehyde must be added to 175 g of ethanol to give a solution whose boiling point is 82.7°c? kb = 1.22°c/m, boiling point of pure ethanol = 78.5°c

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 12:30
We just started a new lesson in chemistry and everyone hates it and i dont get it one bit. i hate school. h el p.balanced equationc3h8+5o2-> 3co2+4h2o1.) if you start with 14.8g of propane(c3h8) and 3.44g of oxygen, which is the limiting reactant -check my answer 2.)what mass of excess reagent is left over? 3.)what mass of carbon dioxide can be made? 4.)what mass of water is produced?
Answers: 2


Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3

Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2
You know the right answer?
Cinnamaldehyde (mm = 132.15 g/mol) is used as a flavoring agent. what mass of cinnamaldehyde must be...
Questions

Mathematics, 10.10.2019 07:30


History, 10.10.2019 07:30



English, 10.10.2019 07:30


English, 10.10.2019 07:30


Mathematics, 10.10.2019 07:30

History, 10.10.2019 07:30

Mathematics, 10.10.2019 07:30

Mathematics, 10.10.2019 07:30

Mathematics, 10.10.2019 07:30

Biology, 10.10.2019 07:30


History, 10.10.2019 07:30


Chemistry, 10.10.2019 07:30

English, 10.10.2019 07:30

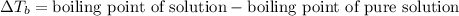
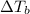 = ? °C
= ? °C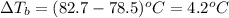
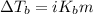
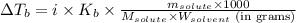
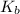 = molal boiling point elevation constant = 1.22°C/m.g
= molal boiling point elevation constant = 1.22°C/m.g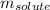 = Given mass of solute (cinnamaldehyde) = ? g
= Given mass of solute (cinnamaldehyde) = ? g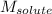 = Molar mass of solute (cinnamaldehyde) = 132.15 g/mol
= Molar mass of solute (cinnamaldehyde) = 132.15 g/mol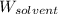 = Mass of solvent (ethanol) = 175 g
= Mass of solvent (ethanol) = 175 g