
Chemistry, 18.09.2019 23:20 MrTeriffic
What mass of hydrogen gas, in mg, is dissolved in 3.15×102 ml of water when the partial pressure of hydrogen above the water is 3.6 atm at 25 °c? the henry's constant for hydrogen gas in water at 25 °c is 7.8×10-4 m/atm.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:30
How air particles exert a pressure on the inside of the balloon
Answers: 1

Chemistry, 22.06.2019 03:00
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1
You know the right answer?
What mass of hydrogen gas, in mg, is dissolved in 3.15×102 ml of water when the partial pressure of...
Questions

Physics, 27.08.2019 01:00

Social Studies, 27.08.2019 01:00

Advanced Placement (AP), 27.08.2019 01:00


Biology, 27.08.2019 01:00

Mathematics, 27.08.2019 01:00


History, 27.08.2019 01:00


Chemistry, 27.08.2019 01:00

History, 27.08.2019 01:00

English, 27.08.2019 01:00





Chemistry, 27.08.2019 01:00

Mathematics, 27.08.2019 01:00


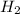 (hydrogen gas), its concentration into the 315 mL of water is given by:
(hydrogen gas), its concentration into the 315 mL of water is given by:
![m=(2.808x10^{-3} \frac{mol H_{2}}{L} )*(0.315L)*(\frac{2g H_{2} }{1 mol H_{2}} )*(\frac{1000 mg H_{2}}{1 g H_{2}} )[tex]m=1.77 mg H_{2}](/tpl/images/0241/1577/7964b.png)


