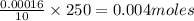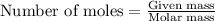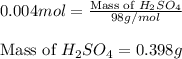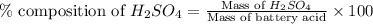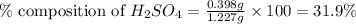Asample of battery acid is to be analyzed for its sulfuric acid content. a 1.00-ml sample weighs 1.227 g . this 1.00-ml sample is diluted to 250.0 ml, and 10.00 ml of this diluted acid requires 35.05 ml of 4.462×10−3 m ba(oh)2 for its titration. what is the mass percent of h2so4 in the battery acid?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Electrons are extremely important to what area of technology? a) anti-aging research b) household product development c) electronics d) drug discovery
Answers: 3

Chemistry, 22.06.2019 11:50
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

Chemistry, 22.06.2019 17:00
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1

Chemistry, 22.06.2019 19:40
What type of electromagnetic waves does the human eye see as the colors red blue or green a visible light waves b radio waves c infrared waves d microwaves
Answers: 1
You know the right answer?
Asample of battery acid is to be analyzed for its sulfuric acid content. a 1.00-ml sample weighs 1.2...
Questions


Mathematics, 21.01.2020 04:31

Computers and Technology, 21.01.2020 04:31





Social Studies, 21.01.2020 04:31








Mathematics, 21.01.2020 04:31




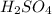 in the battery acid is 31.9 %
in the battery acid is 31.9 %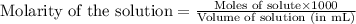
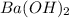 solution =
solution = 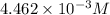
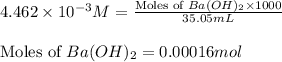
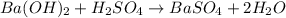
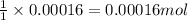 of sulfuric acid.
of sulfuric acid.