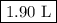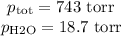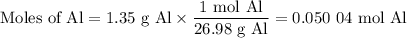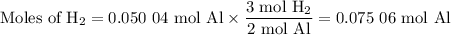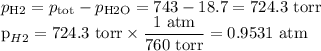Chemistry, 19.09.2019 03:00 davelopez979
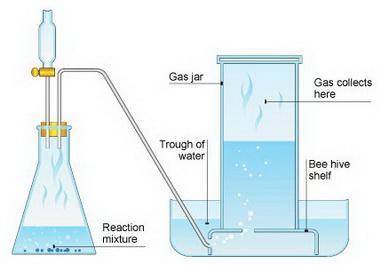
Suppose we now collect hydrogen gas, h2(g), over water at 21◦c in a vessel with total pressure of 743 torr. if the hydrogen gas is produced by the reaction of aluminum with hydrochloric acid:
2al(s) + 6hcl(aq) → 2alcl3(aq) + 3h2(g)
what volume of hydrogen gas will be collected if 1.35 g al(s) reacts with excess hcl(aq)? express
your answer in liters.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:40
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3

Chemistry, 22.06.2019 05:30
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2

Chemistry, 22.06.2019 19:40
What type of electromagnetic waves does the human eye see as the colors red blue or green a visible light waves b radio waves c infrared waves d microwaves
Answers: 1

Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1
You know the right answer?
Suppose we now collect hydrogen gas, h2(g), over water at 21◦c in a vessel with total pressure of 74...
Questions





Health, 07.01.2021 20:40




Biology, 07.01.2021 20:40

English, 07.01.2021 20:40

German, 07.01.2021 20:40







English, 07.01.2021 20:40


Biology, 07.01.2021 20:40