
Chemistry, 19.09.2019 04:30 friendsalwaysbae
Homework 3 write the balanced equations. calculate how many grams of each reactant will be needed to obtain 100.0 grams of the insoluble product formed in the reaction. show complete solutions. 1. aluminum chloride + calcium hydroxide aluminum hydroxide + calcium chloride 2. mercury (ii) oxide mercury + oxygen 3. barium nitrate + copper (ii) sulfate barium sulfate+ copper (ii) nitrate 4. lead (ii) chloride + potassium iodide lead (ii) iodide + potassium chloride 5. sodium sulfide + copper (ii) chloride copper (ii) sulfide + sodium chloride

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 3

Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1
You know the right answer?
Homework 3 write the balanced equations. calculate how many grams of each reactant will be needed to...
Questions












Advanced Placement (AP), 03.09.2019 21:30








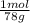 = 1,282 moles of Al(OH)₃.
= 1,282 moles of Al(OH)₃.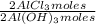 = 1,282 moles of AlCl₃ ×
= 1,282 moles of AlCl₃ × 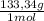 = 170,9 g of AlCl₃
= 170,9 g of AlCl₃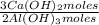 = 1,923 moles of Ca(OH)₂ ×
= 1,923 moles of Ca(OH)₂ × 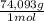 = 142,5 g of Ca(OH)₂
= 142,5 g of Ca(OH)₂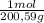 = 0,4985 moles of Hg.
= 0,4985 moles of Hg.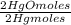 = 0,4985 moles of HgO ×
= 0,4985 moles of HgO × 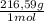 = 108,0 g of HgO
= 108,0 g of HgO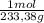 = 0,4285 moles of BaSO₄.
= 0,4285 moles of BaSO₄.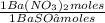 = 0,4285 moles of Ba(NO₃)₂ ×
= 0,4285 moles of Ba(NO₃)₂ × 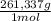 = 112,0 g of Ba(NO₃)₂
= 112,0 g of Ba(NO₃)₂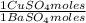 = 0,4285 moles of CuSO₄ ×
= 0,4285 moles of CuSO₄ × 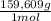 = 68,39 g of CuSO₄
= 68,39 g of CuSO₄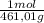 = 0,2169 moles of PbI₂.
= 0,2169 moles of PbI₂.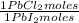 = 0,2169 moles of PbCl₂ ×
= 0,2169 moles of PbCl₂ × 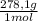 = 60,32 g of PbCl₂
= 60,32 g of PbCl₂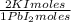 = 0,4338 moles of KI ×
= 0,4338 moles of KI × 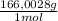 = 72,01 g of KI
= 72,01 g of KI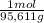 = 1,046 moles of CuS.
= 1,046 moles of CuS.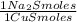 = 1,046 moles of Na₂S ×
= 1,046 moles of Na₂S × 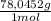 = 81,63 g of Na₂S
= 81,63 g of Na₂S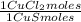 = 1,046 moles of CuCl₂ ×
= 1,046 moles of CuCl₂ × 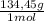 = 140,6 g of CuCl₂
= 140,6 g of CuCl₂

