Chemistry, 19.09.2019 16:30 buddyshaw76
The decomposition of a to b is a first-order reaction with a half-life of 14.2 min: a → 2b if the initial concentration of a is 0.304 m, how long will it take for the concentration of a to decrease by 43.0 %?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1

Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 17:30
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2
You know the right answer?
The decomposition of a to b is a first-order reaction with a half-life of 14.2 min: a → 2b if the i...
Questions





Mathematics, 27.07.2019 19:00

Mathematics, 27.07.2019 19:00


History, 27.07.2019 19:00


English, 27.07.2019 19:10



Mathematics, 27.07.2019 19:10


Biology, 27.07.2019 19:10



Mathematics, 27.07.2019 19:10


![Ln [A] = -k.t + Ln [A]_{0}](/tpl/images/0243/4301/30b90.png)
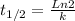
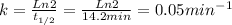
![[A] = \frac{43.0}{100}x[A]_{0}](/tpl/images/0243/4301/7dcf6.png)
![Ln \frac{43.0}{100} [A]_{0} = -k.t + Ln [A]_{0}](/tpl/images/0243/4301/2b903.png)
![(Ln \frac{43.0}{100} [A]_{0} - Ln [A]_{0} ) / -k = t](/tpl/images/0243/4301/1d19e.png)