
Polonium- 210 , po210 , decays to lead- 206 , pb206 , by alpha emission according to the equation po84210⟶pb82206+he24 if the half-life, /2 , of po210 is 138.4 days , calculate the mass of pb206 produced from a 561.0 mg sample of polonium(iv) chloride, pocl4 , that is left untouched for 333.8 days . assume that the only polonium isotope present in the sample is the po210 isotope. the isotopic molar masses of po210 is 209.98 g/mol and pb206 is 205.97 g/mol .

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:00
What stress will shift the following equilibrium system to the left? n2(g) + 3h2(g) ⇌ 2nh3(g) adding more n2(g) adding more nh3(g) increasing the pressure of the system reducing the volume of the container
Answers: 1

Chemistry, 22.06.2019 03:30
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 08:30
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1
You know the right answer?
Polonium- 210 , po210 , decays to lead- 206 , pb206 , by alpha emission according to the equation po...
Questions

Spanish, 29.01.2020 19:42


History, 29.01.2020 19:42



Mathematics, 29.01.2020 19:42


Spanish, 29.01.2020 19:42




History, 29.01.2020 19:42

Mathematics, 29.01.2020 19:42

Mathematics, 29.01.2020 19:42


English, 29.01.2020 19:42

English, 29.01.2020 19:42



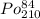 ⟶
⟶ 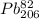 +
+ 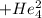
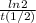
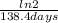 =0,005008 days^(-1)
=0,005008 days^(-1)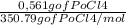 = 0,001599 mol of PoCl4
= 0,001599 mol of PoCl4

