
Acompound of formula xcl3 reacts with aqueous agno3 to yield solid agcl according to the following equation: xcl3(aq)+3agno3(aq)→x(no3)3(aq)+3ag cl(s) when a solution containing 0.521 g of xcl3 was allowed to react with an excess of aqueous agno3, 1.68 g of solid agcl was formed. what is the identity of the atom x?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:20
Much of the general structure and physical properties of the interior of the earth are inferred from: a)deep oil and gas bore holes b)geologic investigations c)analysis of seismic waves d) study of volcanoes
Answers: 1

Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1

Chemistry, 22.06.2019 14:00
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2

You know the right answer?
Acompound of formula xcl3 reacts with aqueous agno3 to yield solid agcl according to the following e...
Questions


Social Studies, 28.09.2020 19:01

Mathematics, 28.09.2020 19:01


Chemistry, 28.09.2020 19:01

Mathematics, 28.09.2020 19:01



Geography, 28.09.2020 19:01

English, 28.09.2020 19:01

Mathematics, 28.09.2020 19:01

Mathematics, 28.09.2020 19:01



Chemistry, 28.09.2020 19:01





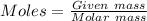
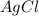 = 1.68 g
= 1.68 g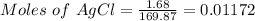
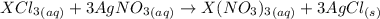
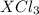 undergoes reaction.
undergoes reaction.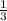 mole of
mole of 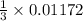 mole of
mole of 

