
An ideal gas in a cylindrical container of radius r and height h is kept at constant pressure p. the bottom of the container is maintained at temperature t0 while the top at temperature t1. assuming a linear temperature distribution along the cylinder, calculate the total mass of gas within the container.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the formula that this ionic compounds could form sr2+p3-o2-
Answers: 3

Chemistry, 22.06.2019 02:00
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3

Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

You know the right answer?
An ideal gas in a cylindrical container of radius r and height h is kept at constant pressure p. the...
Questions



Arts, 07.03.2021 06:30



Chemistry, 07.03.2021 06:30

Mathematics, 07.03.2021 06:30


Mathematics, 07.03.2021 06:30


Health, 07.03.2021 06:30



Mathematics, 07.03.2021 06:30

English, 07.03.2021 06:30

Mathematics, 07.03.2021 06:30


Mathematics, 07.03.2021 06:30


Mathematics, 07.03.2021 06:30



 or
or 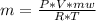 (equation 2)
(equation 2)



