
Chemistry, 19.09.2019 20:30 lulabelles7750
Given the following balanced equation at 120°c: a(g) + b(g) ⇋ 2 c(g) + d(s)(a) at equilibrium a 4.0 liter container was found to contain 1.60 moles of a, and 0.40 moles of b, and 0.40 moles of c, and 1.60 moles of d. calculate kc.(b) if 0.20 moles of b and 0.20 mole of c are added to this system, what will be the new equilibrium concentration of a be? (c) if the volume of the container in which the system is at equilibrium [part (a)] is suddenly halved, what will be the new equilibrium concentrations?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3


Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1

Chemistry, 22.06.2019 19:00
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2
You know the right answer?
Given the following balanced equation at 120°c: a(g) + b(g) ⇋ 2 c(g) + d(s)(a) at equilibrium a 4.0...
Questions

Mathematics, 13.02.2020 00:19

Physics, 13.02.2020 00:19





History, 13.02.2020 00:19




Mathematics, 13.02.2020 00:19


Spanish, 13.02.2020 00:20

Mathematics, 13.02.2020 00:20

Mathematics, 13.02.2020 00:20

Mathematics, 13.02.2020 00:20




![\frac{[C]^2}{[A][B]}](/tpl/images/0244/0592/0c73c.png)
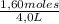 = 0,4 M
= 0,4 M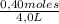 = 0,1 M
= 0,1 M![\frac{[0,1]^2}{[0,4][0,1]}](/tpl/images/0244/0592/6198c.png) = 0,25
= 0,25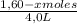
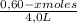
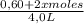
![\frac{[0,60+2x]^2}{[1,60-x][0,60-x]}](/tpl/images/0244/0592/06e92.png)
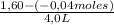 = 0,41 M
= 0,41 M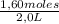 = 0,8 M
= 0,8 M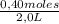 = 0,2 M
= 0,2 M

