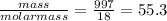Liquids and solids are left out of the equilibrium constant expression because their concentrations remain constant during reactions. what is the molarity concentration of liquid water at 25.0 latex: ^\circ ∘c given that its density is 0.997 g/ml at that temperature?
a.23.5 m
b.0.997 m
c.0.180 m
d.0.156 m
e.55.3 m

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1


Chemistry, 23.06.2019 01:30
Polar bears give birth and hunt on sea ice. which of the following would polar bears survive during the melting of arctic ice? growing another layer of fur during summer migrate inland to search for different food sources staying put until the ice refreezes sticking to the usual diet of seals
Answers: 1

Chemistry, 23.06.2019 06:30
What type of chemical reaction occurs between silver nitrate (agno3) and copper (cu)? the equation i was given is 2agno3 + cu —> 2ag+ cu(no3)2.
Answers: 1
You know the right answer?
Liquids and solids are left out of the equilibrium constant expression because their concentrations...
Questions

Advanced Placement (AP), 04.02.2021 23:40

Mathematics, 04.02.2021 23:40

Mathematics, 04.02.2021 23:40

Social Studies, 04.02.2021 23:40

Mathematics, 04.02.2021 23:40

Mathematics, 04.02.2021 23:40

Mathematics, 04.02.2021 23:40

History, 04.02.2021 23:40



English, 04.02.2021 23:40

Mathematics, 04.02.2021 23:40


Mathematics, 04.02.2021 23:40


Mathematics, 04.02.2021 23:40


Mathematics, 04.02.2021 23:40