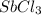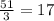Chemistry, 19.09.2019 20:30 milkshakegrande101
Abinary ionic compound is known to contain a cation with 51 protons and 48 electrons. the anion contains one-third the number of protons as the cation. the number of electrons in the anion is equal to the number of protons plus 1. what is the formula of this compound? what is the name of this compound?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 13:30
What is the molecular formula of a hydrocarbon with m+ = 166? (write the formula with no subscripts, e.g. c4h10.) what is the sum of rings and double bonds in this compound?
Answers: 1


Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 11:30
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2
You know the right answer?
Abinary ionic compound is known to contain a cation with 51 protons and 48 electrons. the anion cont...
Questions

Mathematics, 24.07.2019 10:00

Mathematics, 24.07.2019 10:00

Social Studies, 24.07.2019 10:00


Biology, 24.07.2019 10:00

Biology, 24.07.2019 10:00


History, 24.07.2019 10:00


Mathematics, 24.07.2019 10:00

Social Studies, 24.07.2019 10:00

Social Studies, 24.07.2019 10:00

Mathematics, 24.07.2019 10:00

Mathematics, 24.07.2019 10:00

Social Studies, 24.07.2019 10:00


History, 24.07.2019 10:00

History, 24.07.2019 10:00