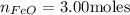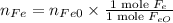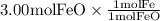Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 13:00
Asolution has a ca2+ concentration of 0.049 m and an f- concentration is 0.147 m at equilibrium. the value of ksp for caf2 at 25°c is 4.0 x 10-11. will this solution form a precipitate? yes no
Answers: 3

Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3

Chemistry, 22.06.2019 15:20
Water is initially present in a state where its molecules are far apart. during a change of state, its molecules slow down. which change of state has most likely taken place? from a gas to a liquid from a liquid to a gas from a solid to a liquid from a gas to a plasma
Answers: 1

Chemistry, 22.06.2019 21:30
What is another way to determine mass times acceleration?
Answers: 1
You know the right answer?
The reaction between iron(ii) oxide and carbon monoxide produces iron and carbon dioxide. how many m...
Questions

English, 18.10.2020 04:01

English, 18.10.2020 04:01





Computers and Technology, 18.10.2020 04:01



Geography, 18.10.2020 04:01







Social Studies, 18.10.2020 04:01



Mathematics, 18.10.2020 04:01