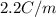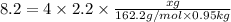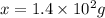When 282 gr of glycine are dissolved in 950 gr of a certain mystery liquid, the freezing point of the solution is 8.2 c less than the freezing point of pure. calculate the mass of iron(iii) chloride that must be dissolved in the same mass of to produce the same depression in freezing point. the van't hoff factor for iron(iii) chloride in x. be sure your answer has a unit symbol, if necessary, and round your answer to significant digits.

Answers: 2
Another question on Chemistry




Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1
You know the right answer?
When 282 gr of glycine are dissolved in 950 gr of a certain mystery liquid, the freezing point of th...
Questions

History, 09.04.2020 22:38

Mathematics, 09.04.2020 22:38




History, 09.04.2020 22:38



Mathematics, 09.04.2020 22:38





Biology, 09.04.2020 22:38

Mathematics, 09.04.2020 22:38

Biology, 09.04.2020 22:38

Mathematics, 09.04.2020 22:38



Spanish, 09.04.2020 22:38

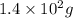
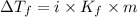
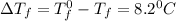 = Depression in freezing point
= Depression in freezing point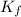 = freezing point constant = ?
= freezing point constant = ?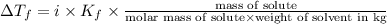
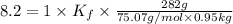
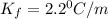
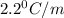
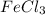 )
)