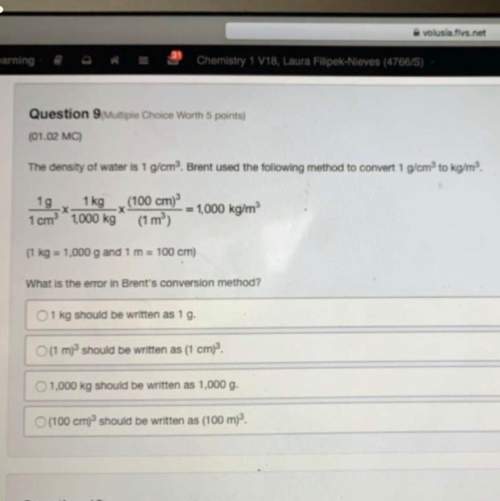
Nitric acid is produced commercially by the ostwald process, represented by the following equations. 4 nh3(g) + 5 o2(g) 4 no(g) + 6 h2o(g) 2 no(g) + o2(g) 2 no2(g) 3 no2(g) + h2o(l) 2 hno3(aq) + no(g) what mass in kg of nh3 must be used to produce 7.6 ✕ 106 kg hno3 by the ostwald process, assuming 100% yield in each reaction?

Answers: 1
Another question on Chemistry

Chemistry, 23.06.2019 00:30
Which radioisotope is used to date fossils? a. oxygen-16 b. carbon-14 c. uranium-238 d. carbon-12
Answers: 2



Chemistry, 23.06.2019 06:30
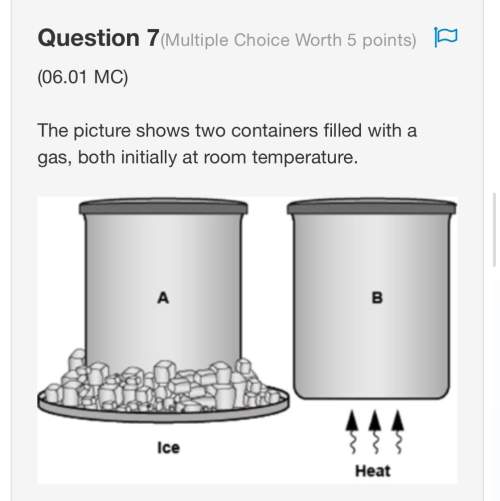
Aplanet similar to earth has four moons roughly the same distance away. the moon that will most affect tides on the planet is the one that has the greatest a) mass. b) volume. c) density. d) amount of water.
Answers: 1
You know the right answer?
Nitric acid is produced commercially by the ostwald process, represented by the following equations....
Questions


Chemistry, 18.09.2019 03:30

Chemistry, 18.09.2019 03:30

English, 18.09.2019 03:30

Computers and Technology, 18.09.2019 03:30

Mathematics, 18.09.2019 03:30

Mathematics, 18.09.2019 03:30


Mathematics, 18.09.2019 03:30

Biology, 18.09.2019 03:30

Mathematics, 18.09.2019 03:30

Mathematics, 18.09.2019 03:30

Computers and Technology, 18.09.2019 03:30






Health, 18.09.2019 03:30

Mathematics, 18.09.2019 03:30