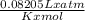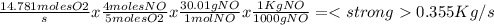Chemistry, 20.09.2019 17:10 DrizzyN2899
Nitric acid is a key industrial chemical, largely used to make fertilizers and explosives. the first step in its synthesis is the oxidation of ammonia. in this reaction, gaseous ammonia reacts with dioxygen gas to produce nitrogen monoxide gas and water.
suppose a chemical engineer studying a new catalyst for the oxidation of ammonia reaction finds that 645. liters per second of dioxygen are consumed when the reaction is run at 195.oc and 0.88 atm. calculate the rate at which nitrogen monoxide is being produced. give your answer in kilograms per second. be sure your answer has the correct number of significant digits.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:00
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2

Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2

Chemistry, 22.06.2019 19:00
Sum of brother and sisters age is 26. four times the brothers age is subtracted from three times the sisters age, the difference is 8. what are the ages of the brother and sister?
Answers: 1

Chemistry, 22.06.2019 22:30
Draw the aromatic compound toluene (methylbenzene). show all hydrogen atoms, including those on the ring.
Answers: 1
You know the right answer?
Nitric acid is a key industrial chemical, largely used to make fertilizers and explosives. the first...
Questions




Mathematics, 26.08.2019 00:10



History, 26.08.2019 00:10

History, 26.08.2019 00:10



Computers and Technology, 26.08.2019 00:10




Mathematics, 26.08.2019 00:10


Biology, 26.08.2019 00:10

Mathematics, 26.08.2019 00:10


Biology, 26.08.2019 00:10