
Chemistry, 20.09.2019 17:30 student0724
Akcl solution is prepared by dissolving 40.0 g kcl (molar mass = 74.55 g/mol) in 250.0 g of water (molar mass = 18.01 g/mol) at 25°c. what is the vapor pressure of the solution if the vapor pressure of water at 25°c is 23.76 mm hg?
a) 20.5 mm hg
b) 22.1 mm hg
c) 22.9 mm hg
d) 24.7 mm hg

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Which position represents spring in the southern hemisphere? a) b) c) d)
Answers: 2


Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1

Chemistry, 23.06.2019 02:00
Anitrogen atom and an oxygen atom combine chemically to form nitric oxide. what is nitric oxide?
Answers: 1
You know the right answer?
Akcl solution is prepared by dissolving 40.0 g kcl (molar mass = 74.55 g/mol) in 250.0 g of water (m...
Questions






Computers and Technology, 17.10.2019 00:30

Biology, 17.10.2019 00:30




Biology, 17.10.2019 00:30





Biology, 17.10.2019 00:30

Biology, 17.10.2019 00:30



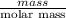
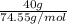
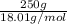
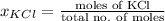
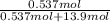
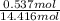
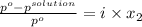
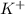 and
and 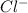 .
.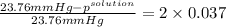
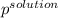 = 22.1 mm Hg
= 22.1 mm Hg


